Sono-Breech
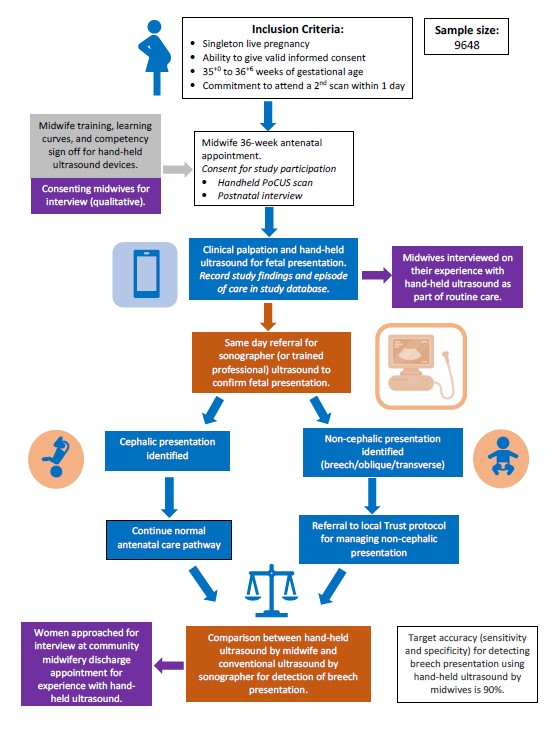
The Sono-breech study is a multi-centre study taking place in at least 13 hospitals across the UK, aiming to determine the diagnostic accuracy of handheld ultrasound at 36-weeks of gestation to determine fetal presentation.
Study Information
For this study, midwives will be provided with PoCUS devices and will undergo special training on how to use them.
At a routine 36-week antenatal appointment the midwife will perform a scan to check the baby’s position using the PoCUS device, in addition to palpating the woman’s abdomen. These women will then have a further conventional ultrasound scan with a sonographer or doctor, so that we can compare the findings with those from the handheld ultrasound scan.
The acceptability of handheld ultrasound will be explored through recorded interviews with selected women and midwives. Those who participate will also be asked to complete questionnaires after their birth, so that we can work out how cost-effective it would be to introduce the scan into routine antenatal care.
We will invite around 10,000 women of different backgrounds from our collaborating maternity units which are geographically diverse and include the North of England, the Midlands, South Coast, London and the East of England to take part in this study.
The team running the study include experts in trial design and statistics (Centre for Trials Research, Cardiff University), obstetricians, midwives, sonographers, neonatologists and health economists from a number of institutions with direct patient involvement.
Consent form
Please find here the consent form. Please note it needs to be filled in with a midwife during an appointment and cannot be done in advance.
Sono-breech Participant Consent Form Word and REDCap screenshot v1.0 22May2024
Eligibility criteria
- Live, singleton pregnancy
- 35+0-36+6 weeks gestation
- Ability to give valid, informed consent
- Commitment to attend second scan within one day
Three different types of handheld PoCUS device are being used in the Sono-breech study:
- GE VScan Air
- Philips Lumify
- Clarius C3HD
The devices are equal and are not being compared. Each one will be connected to an e-tablet (such as an iPad) or mobile phone by Bluetooth. All the devices are safe for both you and your baby.
Birth data
Information about the birth, including the type of delivery and how the baby is doing initially after birth, will be recorded in the same secure database. This data will be collected for up to 28 days following the birth.
Questionnaire
Around six weeks after the birth, two online questionnaires will be sent to participants via email. The questionnaires will ask some questions about the pregnancy and baby. If a participant does not have access to email, a paper questionnaire will be posted.
Interview
Between 6-12 weeks after the birth, some women will be invited to take place in a short interview, where a member of the research team will ask some questions about how they felt about the study and the use of the handheld ultrasound devices. We would also like to interview some women who choose not to take part in the study, to explore why they preferred not to have the scan. Participants can decline to take part in the interview. The interview will be recorded for transcription (carried out by a member of the research team) and the recording will be deleted once this is complete.

